Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
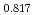 Pu
Pu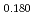 Am
Am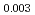 O
O uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 K
uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 KVauchy, R.; Sunaoshi, Takeo*; Hirooka, Shun; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Materials, 580, p.154416_1 - 154416_11, 2023/07
Times Cited Count:4 Percentile:98.08(Materials Science, Multidisciplinary)
Watanabe, Masashi; Kato, Masato
Frontiers in Nuclear Engineering (Internet), 1, p.1082324_1 - 1082324_9, 2023/01
Since the oxygen potential and the oxygen coefficient of UO have a significant impact on fuel performance, many experimental data have been obtained. However, experimental data of the oxygen potential and the oxygen diffusion coefficient in the high temperature region above 1673 K are very limited. In the present study, we aimed to obtain these data and analyze them by defect chemistry. The oxygen potentials and the oxygen chemical diffusion coefficient of UO
have a significant impact on fuel performance, many experimental data have been obtained. However, experimental data of the oxygen potential and the oxygen diffusion coefficient in the high temperature region above 1673 K are very limited. In the present study, we aimed to obtain these data and analyze them by defect chemistry. The oxygen potentials and the oxygen chemical diffusion coefficient of UO were measured by the gas equilibrium method in the near stoichiometric region at temperatures ranging from 1673 to 1873 K. A data set of oxygen potentials was made together with literature data and analyzed by defect chemistry. The oxygen potential of UO
were measured by the gas equilibrium method in the near stoichiometric region at temperatures ranging from 1673 to 1873 K. A data set of oxygen potentials was made together with literature data and analyzed by defect chemistry. The oxygen potential of UO was determined as a function of O/U ratio and temperature, and an equation representing the relationship was derived. The oxygen chemical diffusion coefficient values obtained in this study were reasonably close to the literature values. The oxygen partial pressure dependence of the oxygen chemical diffusion coefficients was predicted from the evaluated results of the oxygen potential data, but no clear dependence was observed.
was determined as a function of O/U ratio and temperature, and an equation representing the relationship was derived. The oxygen chemical diffusion coefficient values obtained in this study were reasonably close to the literature values. The oxygen partial pressure dependence of the oxygen chemical diffusion coefficients was predicted from the evaluated results of the oxygen potential data, but no clear dependence was observed.
Hirooka, Shun; Yokoyama, Keisuke; Kato, Masato
Proceedings of International Conference on Fast Reactors and Related Fuel Cycles; Sustainable Clean Energy for the Future (FR22) (Internet), 8 Pages, 2022/04
Property studies on Am/Np-bearing MOX were carried out and how the properties influences on the irradiation behaviors was discussed. Both Am and Np inclusions increase the oxygen potential of MOX. Inter-diffusion coefficients obtained by using diffusion couple technique indicate that the inter-diffusion coefficient is larger in the order of U-Am, U-Pu and U-Np. Also, the inter-diffusion coefficients were evaluated to be larger at the O/M = 2 than those of O/M  2 by several orders. The increase of oxygen potential with Am/Np leads to higher vapor pressure of UO
2 by several orders. The increase of oxygen potential with Am/Np leads to higher vapor pressure of UO and the acceleration of the pore migration along temperature gradient during irradiation. The redistributions of actinide elements were also considered with the relationship of the pore migration and diffusion in solid state. Thus, the obtained inter-diffusion coefficients directly influence on the redistribution rate. The obtained properties were modelled and can be installed in a fuel irradiation simulation code.
and the acceleration of the pore migration along temperature gradient during irradiation. The redistributions of actinide elements were also considered with the relationship of the pore migration and diffusion in solid state. Thus, the obtained inter-diffusion coefficients directly influence on the redistribution rate. The obtained properties were modelled and can be installed in a fuel irradiation simulation code.
 and (U,Pu,Am,Np)O
and (U,Pu,Am,Np)O
Hirooka, Shun; Matsumoto, Taku; Kato, Masato; Sunaoshi, Takeo*; Uno, Hiroki*; Yamada, Tadahisa*
Journal of Nuclear Materials, 542, p.152424_1 - 152424_9, 2020/12
Times Cited Count:6 Percentile:60.71(Materials Science, Multidisciplinary)The measurement of oxygen potential was conducted at 1,673, 1,773, and 1,873 K for (U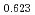 Pu
Pu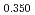 Am
Am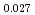 )O
)O and at 1,873 and 1,923 K for (U
and at 1,873 and 1,923 K for (U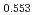 Pu
Pu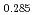 Am
Am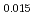 Np
Np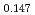 )O
)O by using a thermo-gravimeter and an oxygen sensor. Am inclusion in terms of substituting the U significantly increased the oxygen potential. Similarly, the inclusion of Np as a substitute for U increased the oxygen potential; however, the effect was not as large as that with the Pu or Am addition at the same rate. The results were analyzed via defect chemistry and certain defect formations were suggested in the reducing region and the near-stoichiometric region by plotting the relationship between PO
by using a thermo-gravimeter and an oxygen sensor. Am inclusion in terms of substituting the U significantly increased the oxygen potential. Similarly, the inclusion of Np as a substitute for U increased the oxygen potential; however, the effect was not as large as that with the Pu or Am addition at the same rate. The results were analyzed via defect chemistry and certain defect formations were suggested in the reducing region and the near-stoichiometric region by plotting the relationship between PO and the deviation from the stoichiometry. The equilibrium constants of the defect reactions were arranged to reproduce the experiment such that Am/Np contents were included in the entropy with coefficients fitting the experimental data.
and the deviation from the stoichiometry. The equilibrium constants of the defect reactions were arranged to reproduce the experiment such that Am/Np contents were included in the entropy with coefficients fitting the experimental data.

Suzuki, Kiichi; Kato, Masato; Sunaoshi, Takeo*; Uno, Hiroki*; Carvajal-Nunez, U.*; Nelson, A. T.*; McClellan, K. J.*
Journal of the American Ceramic Society, 102(4), p.1994 - 2008, 2019/04
Times Cited Count:36 Percentile:90.42(Materials Science, Ceramics)The fundamental properties of CeO were assessed using a range of experimental techniques. The oxygen potential of CeO
were assessed using a range of experimental techniques. The oxygen potential of CeO was measured by the thermogravimetric technique, and a numerical fit for the oxygen potential of CeO
was measured by the thermogravimetric technique, and a numerical fit for the oxygen potential of CeO is derived based on defect chemistry. Mechanical properties of CeO
is derived based on defect chemistry. Mechanical properties of CeO were obtained using sound velocity measurement, resonant ultrasound spectroscopy and nanoindentation. The obtained mechanical properties of CeO
were obtained using sound velocity measurement, resonant ultrasound spectroscopy and nanoindentation. The obtained mechanical properties of CeO are then used to evaluate the Debye temperature and Gruneisen constant. The heat capacity and thermal conductivity of CeO
are then used to evaluate the Debye temperature and Gruneisen constant. The heat capacity and thermal conductivity of CeO were also calculated using the Debye temperature and the Gruneisen constant. Finally, the thermal conductivity was calculated based upon laser flash analysis measurements. This result demonstrates that the thermal conductivity has strong dependence upon material purity.
were also calculated using the Debye temperature and the Gruneisen constant. Finally, the thermal conductivity was calculated based upon laser flash analysis measurements. This result demonstrates that the thermal conductivity has strong dependence upon material purity.
 C control rod in severe accident of LWR
C control rod in severe accident of LWRShirasu, Noriko; Kurata, Masaki; Ogawa, Toru*
Proceedings of 2014 Water Reactor Fuel Performance Meeting/ Top Fuel / LWR Fuel Performance Meeting (WRFPM 2014) (USB Flash Drive), 6 Pages, 2014/09
In the accident of Fukushima-Daiichi Nuclear Power Plant, degraded fuels containing Zircaloy probably reacted with B C control blades containing stainless steel cladding or blade sheath. Since light elements like B and C are able to react easily with various elements and form various chemical species, several concerns are pointed out, such as variation in volatility and heat generation by oxidation of B and C. The chemical states of degraded fuel were evaluated on the assumption of thermodynamic equilibrium under various conditions of oxygen potential and temperature. The chemical behavior of B affects significantly the variation in oxygen potential with progressing severe accident, and many kinds of volatile compounds are formed by oxidation. The behavior of B causes the changes of volatility of FPs, such as Sr, Cs and Mo.
C control blades containing stainless steel cladding or blade sheath. Since light elements like B and C are able to react easily with various elements and form various chemical species, several concerns are pointed out, such as variation in volatility and heat generation by oxidation of B and C. The chemical states of degraded fuel were evaluated on the assumption of thermodynamic equilibrium under various conditions of oxygen potential and temperature. The chemical behavior of B affects significantly the variation in oxygen potential with progressing severe accident, and many kinds of volatile compounds are formed by oxidation. The behavior of B causes the changes of volatility of FPs, such as Sr, Cs and Mo.
 Zr
Zr O
O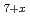
Otobe, Haruyoshi; Nakamura, Akio; Yamashita, Toshiyuki; Minato, Kazuo
Journal of Physics and Chemistry of Solids, 66(2-4), p.329 - 334, 2005/02
Times Cited Count:23 Percentile:65.92(Chemistry, Multidisciplinary)Pyrochlore-type (P-type) zirconias have been attracting significant research interest as disposable forms of high-level radioactive waste. In this work, we have clarified the oxygen potentials (g(O )) vs. oxygen nonstoichiometry (x) and temperature relations of P-type Ce
)) vs. oxygen nonstoichiometry (x) and temperature relations of P-type Ce Zr
Zr O
O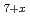 by the emf method and determined the lattice parameters (a0) with respect to x by XRD method.
by the emf method and determined the lattice parameters (a0) with respect to x by XRD method.
Nakajima, Kunihisa; Arai, Yasuo
Journal of Nuclear Materials, 317(2-3), p.243 - 251, 2003/05
Times Cited Count:3 Percentile:25.79(Materials Science, Multidisciplinary)The Knudsen effusion mass-spectrometric measurement of pure UO (s) are carried out at 1673, 1773 and 1873K to evaluate
(s) are carried out at 1673, 1773 and 1873K to evaluate  G
G
 (UO
(UO ,g) as well as to measure the partial pressures of UO
,g) as well as to measure the partial pressures of UO (g) and O
(g) and O (g) over UO
(g) over UO (s) as function of the O/U ratio. It was found that the partial pressures of O
(s) as function of the O/U ratio. It was found that the partial pressures of O (g) over UO
(g) over UO (s) almost agree with the experimental data reported in the past and the values derived from the empirical equation given by Nakamura and Fujino. Further, it was found that the values of
(s) almost agree with the experimental data reported in the past and the values derived from the empirical equation given by Nakamura and Fujino. Further, it was found that the values of  G
G
 (UO
(UO ,g) obtained in this study are in good agreement with the recommended values.
,g) obtained in this study are in good agreement with the recommended values.
 M
M O
O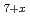 7(M=Pu, Ce) by EMF method
7(M=Pu, Ce) by EMF methodOtobe, Haruyoshi; Nakamura, Akio; Yamashita, Toshiyuki; Ogawa, Toru
Journal of Nuclear Science and Technology, 39(Suppl.3), p.652 - 655, 2002/11
Pyrochlore-type (P-type) zirconia has been widely studied as a disposable form of the high-level radioactive nuclear waste. We have developed the EMF (electromotive force) measurement system using the cubic zirconia sensor, in order to investigate the oxygen potential ( g(O
g(O )) of P-type Zr
)) of P-type Zr Pu(Ce)
Pu(Ce) O
O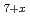 . For P-type Zr
. For P-type Zr Ce
Ce O
O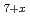 at 1078 K, we have found that
at 1078 K, we have found that  g(O
g(O ) vs. oxygen composition (x) relationship has different behavior between x more than 0.34 and x less than 0.34. This may suggest that the degree and way of the ordering of oxide ions (O
) vs. oxygen composition (x) relationship has different behavior between x more than 0.34 and x less than 0.34. This may suggest that the degree and way of the ordering of oxide ions (O ) and oxygen vacancies (V0) should change around x =0.34. We have also measured the
) and oxygen vacancies (V0) should change around x =0.34. We have also measured the  g(O
g(O ) vs. temperature (T) relationship between 763 and 1078 K at various x, and derived the partial molar enthalpy (h(O
) vs. temperature (T) relationship between 763 and 1078 K at various x, and derived the partial molar enthalpy (h(O )) and entropy (s(O
)) and entropy (s(O )) of oxygen. It was found that h(O
)) of oxygen. It was found that h(O ) and s(O
) and s(O ) of P-type Zr
) of P-type Zr Ce
Ce O
O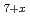 exhibit significantly different behavior from those of fluorite-type CeO
exhibit significantly different behavior from those of fluorite-type CeO . The similar experiments are in progress for P-type Zr
. The similar experiments are in progress for P-type Zr Pu
Pu O
O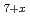 , and the results are discussed in comparison with those of P-type Zr
, and the results are discussed in comparison with those of P-type Zr Ce
Ce O
O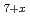 mentioned above.
mentioned above.
Nakajima, Kunihisa; Omichi, Toshihiko*; Arai, Yasuo
Journal of Nuclear Materials, 304(2-3), p.176 - 181, 2002/08
Times Cited Count:3 Percentile:23.41(Materials Science, Multidisciplinary)The oxygen potentials of two type urania-yttria solid solutions, one is the solid solution expressed as nearly U Y
Y O
O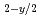 and the other is nearly stoichiometric solid solution, are investigated by use of a mass spectrometer combined with a Knudsen cell. The oxygen potentials for the nearly stoichiometric specimens are much higher than those for pure urania with similar stoichiometry. On the other hand, the oxygen potentials for fully reduced specimens almost agree with those for pure urania having the same oxygen deficiencis.
and the other is nearly stoichiometric solid solution, are investigated by use of a mass spectrometer combined with a Knudsen cell. The oxygen potentials for the nearly stoichiometric specimens are much higher than those for pure urania with similar stoichiometry. On the other hand, the oxygen potentials for fully reduced specimens almost agree with those for pure urania having the same oxygen deficiencis.
Hiura, Nobuo*; Yamaura, Takayuki; Motohashi, Yoshinobu*; Kobiyama, Mamoru*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 1(2), p.202 - 208, 2002/06
The purpose of this study is to develop oxygen sensor which can measure the oxygen potential of the fuel in a nuclear reactor. The oxygen sensor with CaO stabilized zirconia solid electrolyte has been specially designed in order to prolong its life time. Electromotive force (EMF) of solid galvanic cell Ni/NiO|ZrO -CaO|Fe/FeO was measured in both the out-pile tests and the in-situ tests using Japan Material Testing Reactor (JMTR), and the characteristics of EMF was discussed. In the out-pile test, it was found that the EMF was almost equal to the theoretical values at temperatures ranging from 700 to 1,000
-CaO|Fe/FeO was measured in both the out-pile tests and the in-situ tests using Japan Material Testing Reactor (JMTR), and the characteristics of EMF was discussed. In the out-pile test, it was found that the EMF was almost equal to the theoretical values at temperatures ranging from 700 to 1,000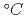 , and the life span of the sensor was very long up to 980h at 800
, and the life span of the sensor was very long up to 980h at 800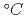 . In the in-situ test, it was found that the EMF showed almost the reliable values up to 300 h (neutron fluence (E
. In the in-situ test, it was found that the EMF showed almost the reliable values up to 300 h (neutron fluence (E  1 MeV) 1.5
1 MeV) 1.5 10
10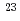 m
m ), at temperatures from 700 to 900
), at temperatures from 700 to 900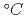 . The imprecision of the EMF was found to be within 6% of the theoretical values up to 1,650 h irradiation time (neutron fluence (E
. The imprecision of the EMF was found to be within 6% of the theoretical values up to 1,650 h irradiation time (neutron fluence (E  1 MeV) 8.0
1 MeV) 8.0 10
10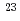 m
m ) at 800
) at 800 . The oxygen sensors were found to be applicable for the oxygen potential measurement of the fuels in a reactor.
. The oxygen sensors were found to be applicable for the oxygen potential measurement of the fuels in a reactor.

Fujino, Takeo*; Sato, Nobuaki*; Yamada, Kota*; Okazaki, Manabu*; Fukuda, Kosaku; Serizawa, Hiroyuki; Shiratori, Tetsuo*
Journal of Nuclear Materials, 289(3), p.270 - 280, 2001/03
Times Cited Count:2 Percentile:19.66(Materials Science, Multidisciplinary)no abstracts in English
 U
U O
O
Fujino, Takeo*; Sato, Nobuaki*; Yamada, Kota*; *; Fukuda, Kosaku; Serizawa, Hiroyuki; Shiratori, Tetsuo
Journal of Nuclear Materials, 265(1-2), p.154 - 160, 1999/00
Times Cited Count:6 Percentile:45.57(Materials Science, Multidisciplinary)no abstracts in English
Takano, Masahide; Minato, Kazuo; Fukuda, Kosaku; Sato, Seichi*; Ohashi, Hiroshi*
Journal of Nuclear Science and Technology, 35(7), p.485 - 493, 1998/07
Times Cited Count:4 Percentile:38.68(Nuclear Science & Technology)no abstracts in English
 , III
, III*; Sato, Seichi*; Ohashi, Hiroshi*; Ogawa, Toru; Ito, Akinori; Fukuda, Kosaku
JAERI-M 92-118, 49 Pages, 1992/08
no abstracts in English
Hidaka, Akihide; Soda, Kunihisa; Sugimoto, Jun; Yamano, N.; Maruyama, Yu
KfK-5108; NEA/CSNI/R(92)10, p.211 - 225, 1992/00
no abstracts in English


 by solid state EMF technique
by solid state EMF techniqueNakamura, Akio; Fujino, Takeo
Journal of Nuclear Materials, 149(1), p.80 - 100, 1987/01
Times Cited Count:44 Percentile:95.53(Materials Science, Multidisciplinary)no abstracts in English


 at relatively small deviation from stoichiometry between 600 and 1400
at relatively small deviation from stoichiometry between 600 and 1400 C
CNakamura, Akio; Fujino, Takeo
Journal of Nuclear Materials, 140, p.113 - 130, 1986/09
Times Cited Count:17 Percentile:83.75(Materials Science, Multidisciplinary)no abstracts in English



 U
U


 O
O

 solid solution
solid solutionUgajin, Mitsuhiro; ; Shiba, Koreyuki
Journal of Nuclear Materials, 116(2-3), p.172 - 177, 1983/00
Times Cited Count:15 Percentile:81.67(Materials Science, Multidisciplinary)no abstracts in English


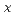 (0≦x≦0.1)
(0≦x≦0.1)Ugajin, Mitsuhiro
Journal of Nuclear Science and Technology, 20(3), p.228 - 236, 1983/00
Times Cited Count:19 Percentile:85.98(Nuclear Science & Technology)no abstracts in English